Background
The therapeutic landscape of mantle cell lymphoma (MCL) has evolved significantly since the early 2000s. We previously reported that a shift in frontline immunochemotherapy choices from 2002−2009 to 2010−2015 was associated with improved event-free survival and overall survival (OS) in both younger (age ≤65) and older (age >65) patients in a prospective Mayo Clinic/University of Iowa cohort (Castellino, Blood Advances, 2022). In addition, we recently reported that changes in treatment for relapsed/refractory (R/R) disease over the last two decades were associated with steady improvements in OS for R/R MCL (Tawfiq, ASH, 2022). In this study, we utilized data from the National Cancer Database (NCDB) to investigate the impact of therapy in the modern era on survival outcomes of patients with MCL in the US since 2004.
Methods
The NCDB is an outcomes database of more than 1,500 hospital-based cancer registries covering approximately 70% of newly diagnosed cancers in the US. The latest version of NCDB was used to identify patients with newly diagnosed MCL from 2004 to 2020. Three treatment eras were defined as Era 1 (2004−2009), Era 2 (2010−2014), and Era 3 (2015−2020), based on immunochemotherapy changes (from Era 1 to Era 2) and increased access to novel agents such as Bruton's tyrosine kinase (BTK) inhibitors (from Era 2 to Era 3). Overall survival between different eras was stratified and analyzed based on 3 age groups: <65, 65−79, and ≥80 years. Survival analysis was performed using the Kaplan-Meier method and all Cox proportional hazards models were adjusted for age and sex.
Results
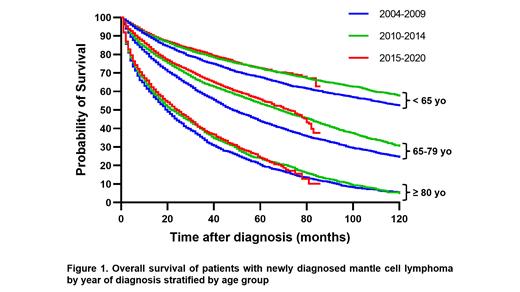
A total of 32,746 patients with newly diagnosed MCL was identified, 12,847 (39.2%) patients were age <65, 14,297 (43.7%) age 65−79, and 5,602 (17.1%) age ≥80. For every age group, the distributions in sex, stage, Charlson-Deyo score, and treatment facility type (academic vs non-academic) were largely similar between eras. As expected, the median OS was significantly worse for patients age ≥80 years (19.8 months) compared to patients age 65-79 years (60.9 months) and patients age <65 years (143.9 months; p <0.001).
In patients age <65 years, OS in Era 2 was improved when compared to Era 1, with a 5-year OS rate of 72.1% vs 67.6% (logrank p<0.001) (Figure 1) and an age- and sex-adjusted hazard ratio (HR) of 0.83 (95% CI 0.78−0.89, p<0.001). OS in Era 3 was similar to that in Era 2, with a 5-year OS rate of 72.2% vs 72.1% (logrank p=0.61) and an age- and sex-adjusted HR of 0.97 (95% CI 0.89−1.06, p=0.51).
In patients age 65-79 years, similar trends in OS changes between eras were observed. The 5-year OS rate in Era 1, Era 2, and Era 3 was 43.3%, 52.8%, and 54.9%, respectively (p<0.001 comparing Era 2 vs Era 1, and p=0.05 comparing Era 3 vs Era 2). Age- and sex-adjusted HR was 0.83 (95% CI 0.78−0.87, p<0.001) comparing Era 2 vs Era 1 and 0.95 (95% CI 0.89−1.01, p=0.07) comparing Era 3 vs Era 2.
In patients age ≥80 years, the improvement in OS from Era 1 to Era 2 was less prominent than other age groups. The 5-year OS rate was 22.9% vs 19.5% (p=0.03), and age- and sex-adjusted HR was 0.88 (95% CI 0.81−0.94, p<0.001). OS in Era 3 vs Era 2 was similar, with a 5-year OS rate of 23.1% vs 22.9% (logrank p=0.79) and an age- and sex-adjusted HR of 0.98 (95% CI 0.91−1.06, p=0.67).
Conclusions
This large NCDB study demonstrated that survival of patients with newly diagnosed MCL has improved over the last two decades. The results are consistent with our Mayo Clinic/University of Iowa prospective cohort study and confirm an improved OS after 2010, which was most likely driven by both frontline immunochemotherapy changes and better access to novel agents for R/R disease. The 5-year OS in patients diagnosed in 2015−2020 did not appear to be superior to those in 2010−2014, despite presumably having better access to novel agents such as BTK inhibitors in the R/R setting. This lack of a difference in OS may be partially due to short follow-up, but may also suggest that further improvement in frontline therapies by incorporating novel agents may be required to improve OS. The OS of patients age ≥80 years is limited and has only minimally improved after 2010, highlighting a significant unmet need for therapies with improved tolerability and efficacy in this age group.
Disclosures
Diefenbach:Beigene: Consultancy, Research Funding; Genmab: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Regeneron: Consultancy, Research Funding; Roche/Genentech: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding. Ruan:AstraZeneca: Consultancy, Research Funding; Secura Bio: Consultancy; Daiichi Sankyo: Research Funding; Genentech: Research Funding; BMS: Research Funding. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Paludo:Karyopharm: Research Funding; Biofourmis: Research Funding; AbbVie: Consultancy. Munoz:Merck: Research Funding; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; Incyte: Research Funding; Lilly/Loxo: Consultancy; Bayer: Consultancy, Research Funding, Speakers Bureau; Celgene/ Bristol-Myers Squibb: Consultancy, Speakers Bureau; Celgene: Research Funding; Pharmacyclics/ Janssen: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; MEI: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Physicians' Education Resource: Honoraria; TG Therapeutics: Consultancy; Targeted Oncology: Honoraria; Portola: Research Funding; Verastem: Consultancy, Speakers Bureau; Alexion: Consultancy; Morphosys/Incyte: Consultancy; Curio: Honoraria; Genmab: Consultancy; ADC Therapeutics: Consultancy; Karyopharm: Consultancy; Epizyme: Consultancy; Millennium: Research Funding; OncView: Honoraria; Beigene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Acrotech/Aurobindo: Consultancy, Speakers Bureau; Kyowa: Honoraria, Speakers Bureau; Pharmacyclics/Abbvie: Consultancy, Research Funding. Ansell:ADC Therapeutics, Affimed, Bristol-Myers Squibb Company, Pfizer Inc, Regeneron Pharmaceuticals Inc, Seagen Inc, Takeda Pharmaceuticals USA Inc.: Other: Contracted Research. Witzig:Karyopharm: Research Funding; Kura Oncology: Research Funding; Salarius Pharma: Membership on an entity's Board of Directors or advisory committees; ADC: Membership on an entity's Board of Directors or advisory committees. Habermann:sorrento: Research Funding; Genentech: Research Funding; BMS: Research Funding. Cerhan:Protagonist: Other: Safety Monitoring Committee; NanoString: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; Genentech: Research Funding. Nowakowski:Genentech: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Incyte: Consultancy; Kite Pharma: Consultancy; MEI Pharma: Consultancy; Kymera Therapeutics: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy; Celgene Corporation: Consultancy; Bantam Pharmaceutical LLC: Consultancy; Curis: Consultancy; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Debiopharm: Consultancy; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seagen: Consultancy; Selvita Inc: Consultancy; Zai Lab Limited: Consultancy. Wang:TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genmab: Research Funding; Genentech: Research Funding; Novartis: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal